Use of polarization resistance measurement for corrosion inhibitor selection.
Question
How to select a corrosion inhibitor for the metal to protect?
Is inhibition maintained over time?
Which is the most effective inhibitor?
Expertise
Materia Nova has extensive experience in using electrochemical techniques to assess the corrosion of materials.
In this case study, the polarization resistance (Rp) method was used to determine the effectiveness of 3 different corrosion inhibitors for galvanized steel substrates. In general, the method is used to monitor the evolution over time of corrosion of a material in a given medium, or the effect of corrosion inhibitors in that medium.
In practice, metal samples are brought into contact with a corrosive solution. The experimental set-up consists of a 3-electrode electrochemical cell. This comprises the working electrode, which is the material to be analyzed, a reference electrode to control the potential of the working electrode, and a counter-electrode to close the circuit. The cell is filled with the chosen corrosive solution.
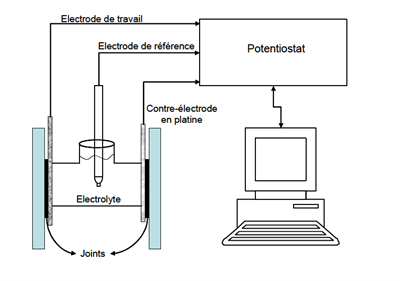
The potentiostat is used to weakly polarize (10 to 20 mV) the material to be analyzed around its resting potential in this solution, and to record the current response. In this way, the measurement is non-destructive, as the metal corrodes "freely", and the same sample can be monitored over time. The higher the polarization resistance, the more resistant the system is to corrosion.
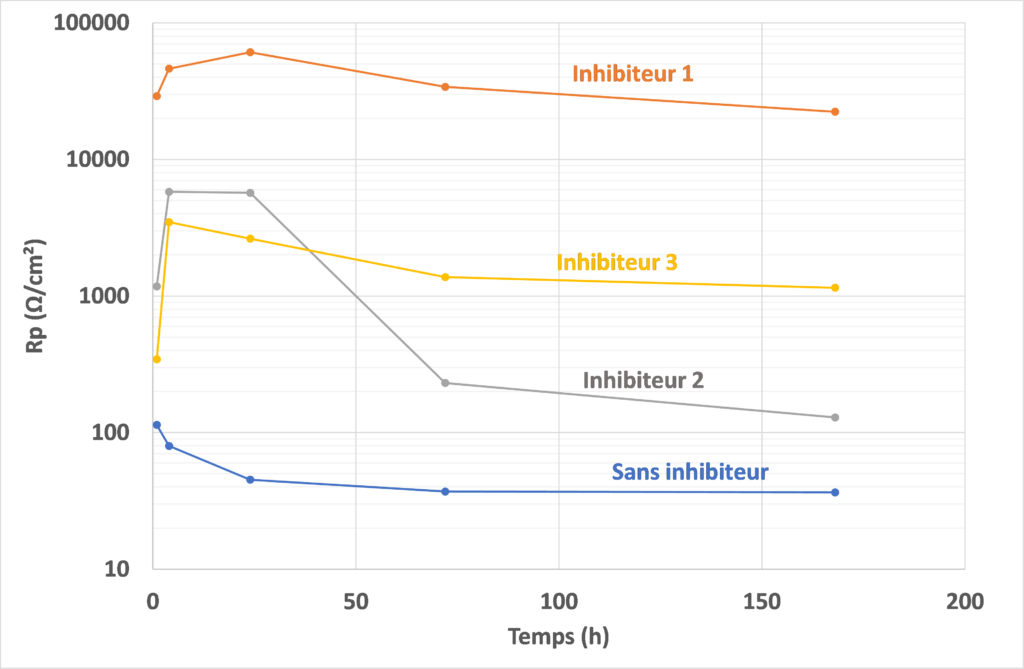

Solution
In the absence of inhibitor, Rp values are low and decrease over time. Zinc-plated steel shows numerous corrosion spots at the end of the test.
In the presence of inhibitor 1, Rp values are very high from the outset and remain so over time. The excellent protection provided by this compound is confirmed by its surface condition at the end of the test: no signs of corrosion are visible. Inhibitors 2 and 3 provide less protection than the former. Compound 2 is effective for up to 24 hours, after which the Rp value falls sharply, showing the loss of the protection initially provided. Zinc-coated steel shows numerous corrosion spots at the end of the test, but in lesser quantities than without inhibitor. Finally, compound 3 shows lower Rp values than compound 1, but these are maintained over time. This shows that the protection provided is maintained, with zinc-coated steel showing only a few spots of corrosion.
The technique can therefore be used to select the most effective corrosion inhibitors. By adjusting the inhibitor concentration in the solution, it is also possible to determine their effectiveness threshold.
Complementary surface analyses can reveal the formation of a protective layer where appropriate.
